Communication Solution for Pharmaceutical Plants, Clean Rooms
Why Choose Our Pharmaceutical & Clean Room Communication Solutions?
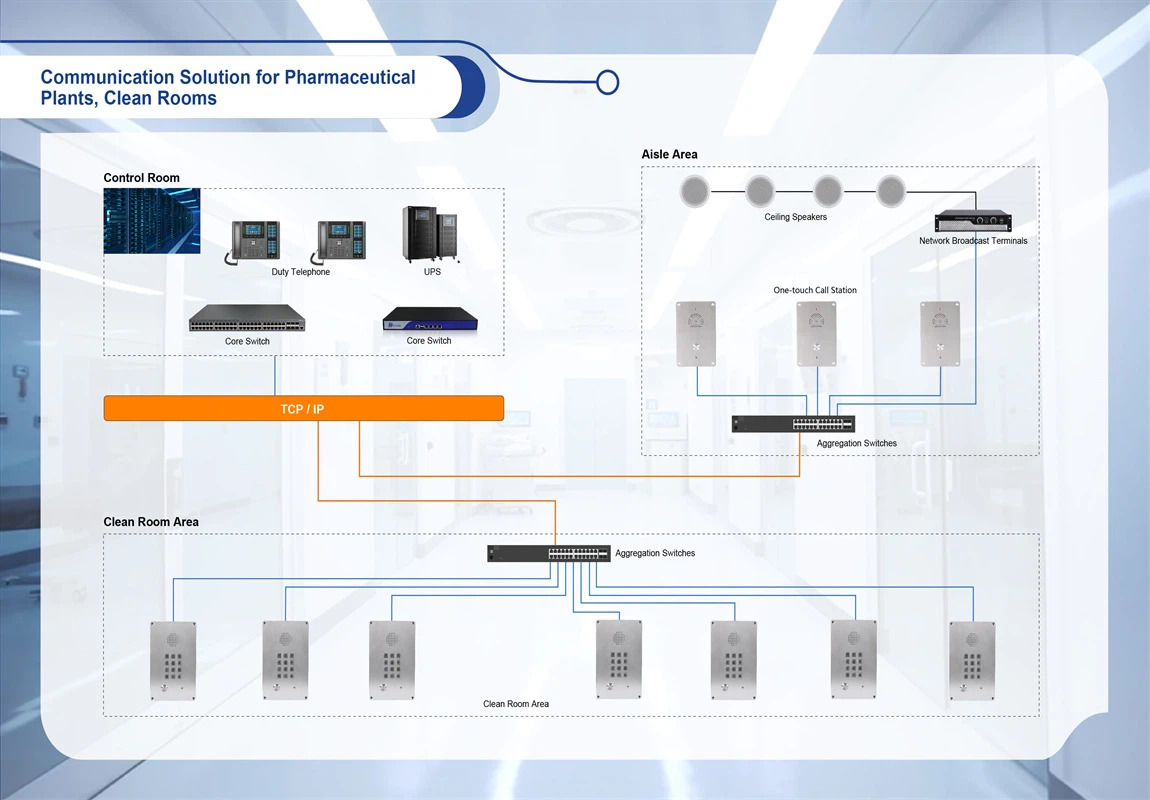
Pharmaceutical plants and clean room environments present unique communication challenges that require specialized solutions designed to maintain sterile conditions while ensuring reliable, high-quality communication. These controlled environments demand communication systems that comply with strict regulatory requirements, maintain contamination control, and provide crystal-clear audio transmission despite the challenges posed by protective equipment and controlled atmospheric conditions.
Our advanced communication solutions are specifically engineered for pharmaceutical manufacturing facilities, clean rooms, and sterile processing environments. These systems integrate seamlessly with existing facility infrastructure while maintaining the stringent cleanliness and safety standards required in pharmaceutical production. Our solutions ensure compliance with Good Manufacturing Practice (GMP) regulations and other pharmaceutical industry standards.
With specialized features including acoustic echo processing, flush-mount installations, and sterile-environment compatibility, our communication systems provide pharmaceutical facilities with the reliable, contamination-free communication infrastructure essential for safe operations, emergency response, and regulatory compliance in critical manufacturing environments.
Key Features & Capabilities
- Advanced Acoustic Echo Processing: The telephone is equipped with an acoustic echo processing system, so you can enjoy high-quality calls whether in public areas or noisy environments, ensuring clear communication even when wearing protective equipment or in controlled atmosphere conditions.
- Comprehensive Communication Modes: Based on the on-site environment, provide users with audio intercom, video linkage, SIP calls, call recording, and data storage traceability management, offering complete communication flexibility for various pharmaceutical operational requirements.
- Flush Mount Emergency Communication: Install flush mounting emergency telephone communication equipment in the clean room. You can quickly call the control center by dialing the preset emergency number, ensuring rapid response while maintaining sterile environment integrity.
- Sterile Environment Compliance: All communication equipment is designed and manufactured to meet pharmaceutical industry standards for clean room environments, including smooth surfaces, minimal particle generation, and easy cleaning protocols compatible with pharmaceutical sanitization procedures.
- GMP Regulatory Compliance: Communication systems are designed to meet Good Manufacturing Practice (GMP) requirements, including documentation capabilities, audit trails, and validation support necessary for pharmaceutical regulatory compliance.
- Contamination-Free Installation: Specialized flush-mount and sealed installation methods ensure that communication equipment does not compromise clean room classifications or introduce contamination risks to sterile pharmaceutical processes.
- High-Quality Audio in PPE Environments: Advanced audio processing technology ensures clear communication even when personnel are wearing protective equipment, face masks, respirators, or working within laminar flow environments.
- Emergency Response Integration: Dedicated emergency communication protocols with direct connections to control centers, safety systems, and emergency response teams, ensuring immediate assistance during critical pharmaceutical manufacturing incidents.
- Hands-Free Operation: Voice-activated and hands-free communication options enable personnel to maintain sterile protocols while communicating, preventing contamination from manual equipment operation.
- Multi-Zone Communication: Comprehensive communication coverage across different clean room classifications (ISO 5, ISO 7, ISO 8) with appropriate equipment specifications for each environmental classification level.
- Digital Recording and Traceability: Complete digital recording capabilities with time-stamping, user identification, and secure data storage for regulatory compliance, quality assurance, and batch record documentation requirements.
- Integration with Process Control Systems: Seamless integration with pharmaceutical manufacturing execution systems (MES), building management systems, and process control infrastructure for comprehensive facility management.
- Antimicrobial Surface Treatments: Communication equipment features antimicrobial surface treatments and materials resistant to frequent sanitization with pharmaceutical-grade cleaning agents and disinfectants.
- Environmental Monitoring Integration: Communication systems can integrate with environmental monitoring systems to provide alerts about temperature, humidity, pressure differentials, and particle count excursions in real-time.
- Batch Production Communication: Specialized communication protocols for batch manufacturing operations, including shift handover communications, batch record documentation, and production status updates.
- Quality Control Communication: Dedicated communication channels for quality control personnel, including sample tracking, test result communication, and deviation reporting capabilities.
- Maintenance Communication Protocols: Specialized communication systems for maintenance activities in sterile environments, including work permit coordination, contamination prevention protocols, and post-maintenance verification procedures.
- Multi-Language Support: Communication systems support multiple languages to accommodate diverse pharmaceutical workforce environments while maintaining clear understanding of critical safety and operational communications.
- Backup Power Integration: Uninterruptible power supply integration ensures continuous communication capability during power outages, critical for maintaining pharmaceutical production continuity and emergency response capabilities.
- Validation Documentation Support: Complete validation documentation packages including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) to support pharmaceutical facility validation requirements.










